Photoelectric Effect is the
phenomenon that when light shines on a metal surface, electrons are emitted
from the surface.
Photoelectric Effect – A photon
may knock an electron out of an atom and in the process itself disappear.
Electrons should be emitted when
light shines on a metal is consistent with the electromagnetic wave theory of
light – that is the electric field of an EM wave could exert a force on
electrons in the metal and eject some of them
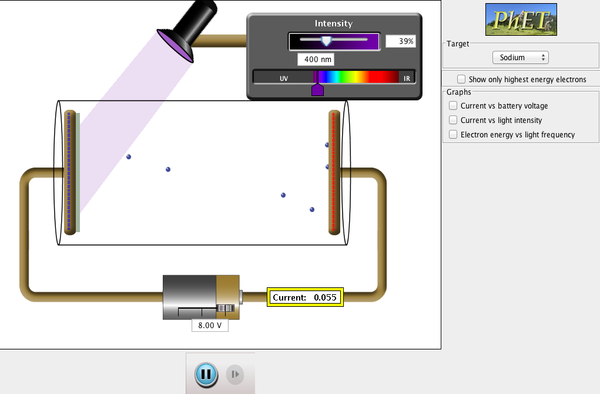
The process of measuring maximum
kinetic energy can be done by using a variable voltage source and reversing the
terminals so that the electrode C is negative and P is positive. The electrons emitted from P will be
repelled by the negative electrode. But if this reverse voltage is small
enough, the fastest electrons will still reach C and there will be a current in
the circuit.
If the reverse voltage is
increase, a point is reached where the current reaches zero, so no electrons
have sufficient energy to reach C. This called stopping potential or stopping
voltage
Wave theory
Assume a monochromatic light. Two
important properties of light are intensity and frequency. When two properties
varied, the wave theory make prediction as below:
·
If the light intensity increase, numbers of
electrons ejected and their maximum
kinetic energy should be increased because – the higher the intensity,
greater electric field amplitude and greater electric field should eject
electrons with higher speed.
·
The frequency of the light should not affect the
kinetic energy of the ejected electrons.
Photon theory
In monochromatic beam, all
photons have the same energy, E=hf.
Increasing the intensity of light mean increasing the number of photons.
- · If the frequency remains the same, it does not affect the energy of each photon
- · An electron is ejected from the metal by a collision with a single photon. Consequently, all the photon energy is transferred to the electron and the forces some minimum energy W0 (Work Function) is required to get an electron out through the surface.
- · hf<W0 - The photon will not have enough energy to eject any electron
- · hf>W0 – The electrons will be ejected and energy will be conserved.
Consideration of photon theory:
- An increase in intensity of the light beams means more photons are incident, so more electrons will be ejected.
- Since the energy of each photon is not changed, the maximum kinetic energy of electrons is not changed by increase in intensity
- If the frequency is increased, the maximum kinetic energy of the electrons increases linearly.
Compton Effect
Compton Effect – a photon
can be scattered from an electron and in the process, lose some energy. But the
photon is not slowdown; it still
travels with speed, c but its frequency will be lower.
Compton scattered short
wavelength light, which is X-rays, from various materials. He found that the
scattered light had a slightly longer wavelength than the incident light,
therefore, there is a slight lower
frequency indication loss of energy.
Since the photon is relativistic
particle that travel with the speed of light, v= c, the momentum of
photon is
Figure 1: Compton Scattering
Atomic
Structure
In 1911, Ernest Rutherford (1871-1937)
theorized that the atom must consist of a
tiny but massive positively charged nucleus, surrounded by electrons some distance away. The electrons would be moving in orbits
about the nucleus.
 |
| Figure 1: Atomic Structure |
Line Spectrum of Hydrogen Atom
Figure 2: Hydrogen Atom
Hydrogen is simplest atom that has only one
electron orbiting its nucleus. It atomic number is 1.
In 1885, J. J. Balmer showed that the four visible
lines in the hydrogen spectrum (with wavelength 656 nm, 486 nm, 434 nm and 410
nm) fit the following formula
Figure 3: Electron transitions for the Hydrogen atom
Wave-Particle Duality
Some indicate that
light behaves like waves and the
other indicates light behaves like stream
of particles. These behaviours of light come in to conclusion as wave-particle
duality.
In 1923, Louis de
Broglie suggests that the wavelength of a particle would be related to its
momentum as in the same way with photon.
p
=
linear momentum
Sometimes it is called
the de
Broglie wavelength of a particle
Video 2: Waves-Particle Duality
Diffraction
of X-Ray
X-ray is produced when electrons accelerated
by a high voltage strike the metal
target inside the X-ray tube. W. C. Roentgen in 1895 discovers the X-ray using
voltages of 30kV – 150 kV. H-rays scattered from a crystal did indeed show the peaks and valleys of a diffraction pattern. It was shown that
X-rays have a wave nature and the
atoms are arranged in a regular way in crystals (serve as diffraction) Today,
X-rays are recognized as electromagnetic
radiation.
The
diffraction of X-rays with wavelength,
that a
reflection from a crystal as described by Bragg equation. Strong reflections are
observed at grazing angles  given by,
given by,
 given by,
given by,
d = distance between reflecting planes in the crystal














No comments:
Post a Comment